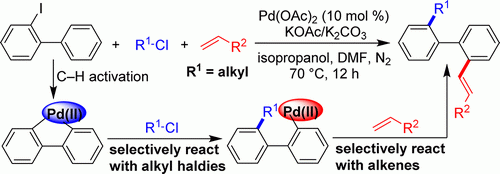
Sequential Difunctionalization of 2-Iodobiphenyls by Exploiting the Reactivities of a Palladacycle and an Acyclic Arylpalladium Species
2016
期刊
Organic Letters
- 卷 18
- 期 9
- 页码 2130-2133
- American Chemical Society (ACS)
- ISSN: 1523-7060
- DOI: 10.1021/acs.orglett.6b00753
